Cell spreading and invasion are fundamental to embryonic development, tissue regulation and regeneration, and the progression of many diseases, including cancer; yet the mechanisms that drive populations of cells to invade distinct targets are poorly understood. We develop mathematical models, in collaboration with experimental researchers, to better understand cell spreading and invasion.
Cranial neural crest cell migration
The embryonic vertebrate neural crest is a multipotent, highly migratory population of cells that contributes to a variety of tissues in different parts of the body, for example, the peripheral nervous system and structures in the head and heart. It provides the most remarkable example of long distance cell invasion that is accessible to manipulation and in vivo observation, and serves as a model system to study both diverse cell invasion mechanisms and a range of birth defects; failure of cranial neural crest invasion results in significant morphological abnormalities of the face, neck and cardiovascular system. The neural crest also provides a model system to study aspects of cancer emergence and treatment, such as metastasis and tumour reprogramming.
The neural crest displays rich and dynamic heterogeneities at the level of cell morphology and gene expression during invasion. The central questions in the field are to what extent these heterogeneities are required for successful invasion of target sites, and are they generated autonomously, or through cell-cell and cell-environment interactions? We have a long-standing interdisciplinary collaboration with Paul Kulesa (Stowers Institute for Medical Research, USA) aimed at tackling this problem. By using a novel multi-scale discrete-continuum model, in tandem with in vivo dynamic imaging and embryo microsurgery, we have convincingly demonstrated that a cell-induced chemoattractant gradient can only facilitate robust invasion in the presence of cell population heterogeneity. Refinement of the model, together with gene expression profiling, revealed that a subpopulation of cells at the most distal invasive front are the ‘leaders’ of invasion, and that migration can be more efficient with fewer leader cells.
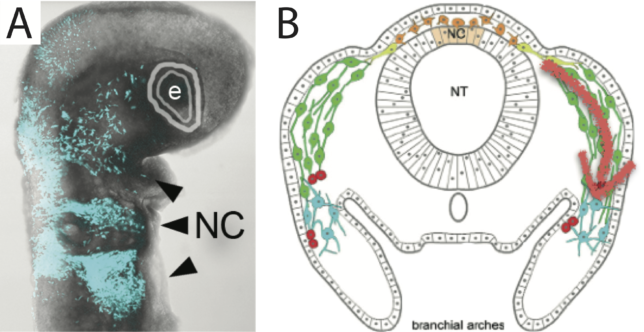
- R. McLennan, C. M. Bailey, L. J. Schumacher, J. M. Teddy, J. A. Morrison, J. C. Kasemeier-Kulesa, L. A. Wolfe, M. M. Gogol, R. E. Baker, P. K. Maini, and P. M. Kulesa (2017). DAN (NBL1) promotes collective neural crest migration by restraining uncontrolled invasion. J. Cell Biol. 216(10). DOI
- L. J. Schumacher, P. M. Kulesa, R. McLennan, R. E. Baker and P. K. Maini (2016). Multidisciplinary approaches to understanding collective cell migration in developmental biology. Open Biol. 6(6):160056. DOI
- R. McLennan, L. J. Schumacher, J. A. Morrison, J. M. Teddy, D. A. Ridenour, A. C. Box, C. L. Semerad, H. Li, W. McDowell, D. Kay, P. K. Maini, R. E. Baker and P. M. Kulesa (2015). VEGF signals induce trailblazer cell identity that drives neural crest migration. Dev. Biol. 407(1):12-25. DOI
- R. McLennan, L. J. Schumacher, J. A. Morrison, J. M. Teddy, D. A. Ridenour, A. C. Box, C. L. Semerad, H. Li, W. McDowell, D. Kay, P. K. Maini, R. E. Baker and P. M. Kulesa (2015). Neural crest migration is driven by a few trailblazer cells with a unique molecular signature narrowly confined to the invasive front. Development 142:2014-2025. DOI
- R. McLennan, L. Dyson, K. W. Prather, J. A Morrison, R. E. Baker, P. K. Maini and P. M. Kulesa (2012). Multiscale mechanisms of cell migration during development: theory and experiment. Development 139:2395-2944. DOI
